Vaccine Storage and Handling
Vaccines and other medical products are essential parts of a healthy society. They provide relief from diseases and suffering. They are designed to improve the quality of life for people. As with any other product, they are manufactured in facilities located near urban centers.
When shipped elsewhere, transportation is provided by a cold chain process in place to safeguard the quality of the medical products. Typically, cold chain operations employ of refrigeration units to maintain ideal conditions for vaccines or other life science products.
Vaccine Cold Chain
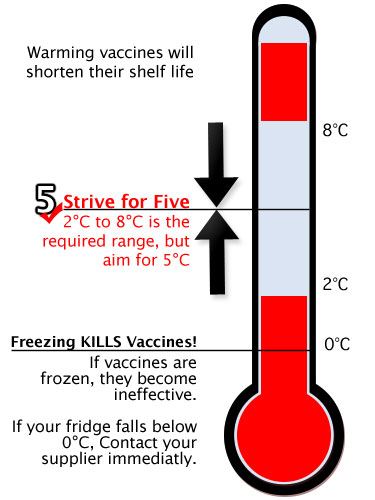
The purpose of the vaccine cold chain is to maintain product quality from the production of the product until the point of administration, by guaranteeing that vaccines are stored and transported within World Health Organization (WHO) recommended temperature ranges.
The cold chain is sometimes also known as the vaccine supply chain, or the immunization supply chain. The cold chain consists of various links that are designed to keep vaccines within WHO-recommended temperature ranges. Following this strategy ensures that cold chain performance is properly monitored and that necessary information is gathered for vaccine quality forecasting.
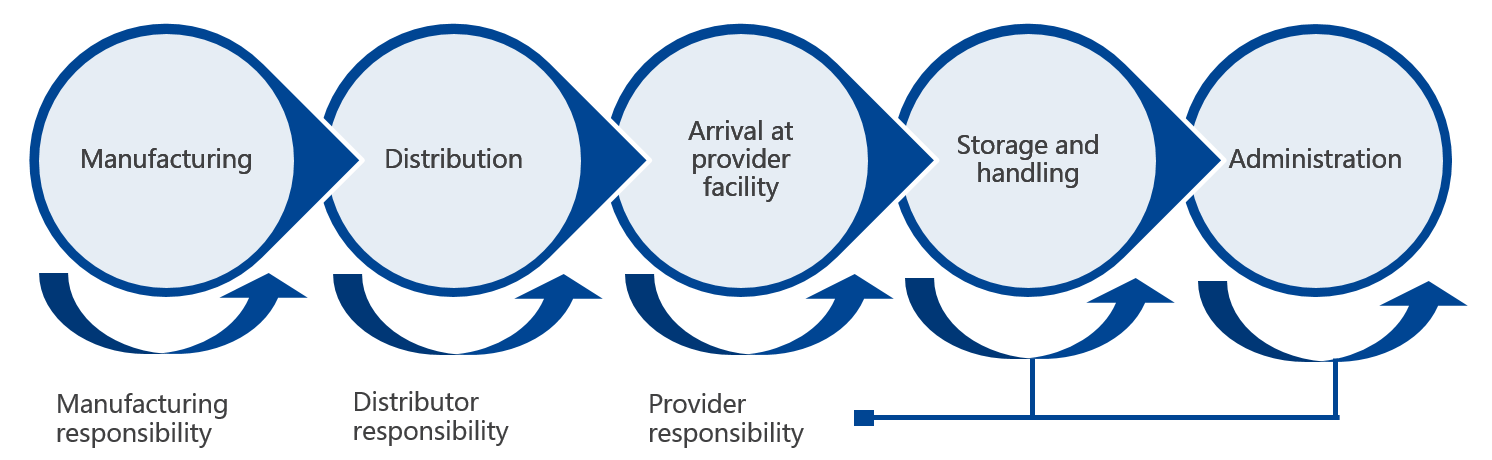
A cold chain is a temperature-controlled supply chain that includes all vaccine-related equipment and procedures. The cold chain begins with the cold storage unit at the manufacturing plant, extends to the transport and delivery of the vaccine and proper storage at the provider facility, and ends with the administration of the vaccine to the patient.
In order to maintain a reliable vaccine cold chain at the optimum level, the following key protocols must be followed:
- Store vaccines and diluents within the required temperature range at all sites
- pack and transport vaccines to and from outreach sites according to recommended procedures
- keep vaccines and diluents within recommended cold chain conditions during immunization sessions.
Temperature requirements for vaccines
Vaccines are biological products that are vulnerable when exposed to conditions outside their safety parameters. Some of them are sensitive to freezing, some to heat, and others to light. Vaccine potency is the ability to protect the vaccinated patient. It can diminish when the vaccine is compromised by temperature excursions. Once potency is lost, it cannot be restored. To preserve its quality, vaccines must be stored away from temperature extremes by using a cold chain that meets specific temperature requirements. It is essential that all those who handle vaccines and diluents know the temperature sensitivities and the recommended storage temperatures for all the vaccines being shipped.
The vaccine cold chain immunization
The vaccines are classified into six categories. Within these six categories, vaccines are arranged in alphabetical order, and not according to their sensitivity to heat. The most heat-sensitive vaccines are in Group A and the least heat-sensitive vaccines are in Group F.
It is worth noting that the heat stability information shown for freeze-dried vaccines applies only to unopened vials. Freeze-dried vaccines can rapidly lose potency if mixed with saline solution. Also, it is imperative to keep opened multi-dose vaccine vials, with no preservative, cooled at temperatures between +2 °C and +8 °C (32 °F and 46.3°F) during the injection session, or within six hours after opening.
Sensitivity to light
There are some vaccines that are sensitive to light and lose potency when exposed to it. They should always be shielded against sunlight or any strong artificial light, and exposure should be minimized. Vaccines that are sensitive to light include Bacille Calmette-Guerin (BCG), measles, measles-rubella, measles-mumps-rubella, and rubella. The vaccines are often stored in dark glass vials that give them some protection from light damage, but they should be kept in their boxes for as long as possible to protect them during storage and transportation.
Controlled Temperature Chain (CTC)
An increasing number of vaccines are tested to see if they are compatibile with a Controlled Temperature Chain (CTC) at ambient temperatures. WHO defines a CTC as the on-label use of a WHO-prequalified vaccine out of the traditional +2 °C and +8 °C (32 °F and 46.3°F) cold chain for a limited period of time with temperature variable of up to 40 °C, just before administration. The CTC approach can be adopted by countries under special circumstances, such as for special strategies, or mass vaccination campaigns at the health center or peripheral health facility level.
As such, health workers can adequately protect vaccines by doing the following:
- Keep vaccines in prescribed vaccine refrigeration equipment.
- Use a temperature monitoring device to ensure temperatures remain between +2 °C and +8 °C (32 °F and 46.3°F).
- Transport vaccines to immunization sessions in a vaccine carrier, correctly packed, using coolant packs where necessary.
- During immunization sessions, fit a foam pad (if available) at the top of the vaccine carrier, to ensure insulation from the outside temperature.
At the health facility, one person will be the supervisor for managing the vaccine cold chain. A second person can be the back-up personnel if the primary person is absent. Their responsibilities are:
- checking and recording vaccine temperatures twice daily; typically, in the morning and at the end of the session or day
- properly storing vaccines, diluents, and water packs
- handling preventative maintenance of the cold chain equipment. All health workers in a facility must have the knowledge to monitor the cold chain and what to do if temperatures are out of range.

Manufacturers, distributors, public health staff, and health care providers are all responsible to ensure the cold chain is maintained once the vaccines are manufactured until they are delivered to the final user.
Vaccine Coordinator
To ensure proper storage and handling of medical products, there should be a primary vaccine coordinator who will be responsible for overseeing the process. An alternate coordinator should be appointed to serve in the absence of the primary coordinator. These individuals should be fully trained in routine and emergency policies and procedures. Coordinator responsibilities may be completed by the coordinator or delegated to appropriate staff. The coordinator must ensure the delegate has documented training, demonstrating competency for the specific tasks assigned and must confirm that tasks are completed.
Some of the coordinator responsibilities include:
- Ordering vaccines
- Supervising proper receipt and storage of vaccine deliveries
- Documenting vaccine inventory information
- Organizing and monitoring vaccines within storage units, including rotating stock and removing expired vaccines
- Setting up temperature monitoring devices (TMDs) and recording daily temperatures
- Responding to temperature excursions (out-of-range temperatures) and equipment failures
- Overseeing vaccine transport (when necessary)
- Overseeing emergency preparations
Vaccine Storage and Handling Standard Operating Procedures (SOPs)
Facilities must develop and maintain clearly written, detailed, and up-to-date storage and handling standard operating procedures (SOPs) recommended by the CDC. SOPs must be familiar with all staff and revised by the vaccine coordinator yearly.
SOPs should contain information for three major areas:
General information – include contact numbers for vaccine manufacturers, equipment service providers, and important facility staff, as well as their job descriptions, regularly used forms, and staff qualifications.
Routine storage and handling – include information on vaccine inventory management, from ordering to monitoring temperature conditions.
Emergency vaccine storage, handling, and transport – procedures to be taken in the event of equipment breakdowns, power failures, natural disasters, or other emergencies that could compromise vaccine storage conditions.
Vaccine Storage and Temperature Monitoring Equipment
It is important for a storage area to have proper monitoring and refrigeration equipment that is set up correctly, maintained appropriately, and repaired as needed. These procedures ensure the protection of patients from receiving compromised vaccines. and prevent the pharma companies against the costs of revaccinating patients, replacing expensive vaccines, and losing brand reputation.
Refrigerators and Freezers
Here are the recommended types of refrigerators and freezers:
Purpose-built or pharmaceutical-grade units designed to either refrigerate or freeze biologics, including vaccines, are used recommended. These units can be compact, under-the-counter style, or larger units.
If a pharmaceutical-grade unit is unavailable, a stand-alone, household unit may be an acceptable option in some practice settings. The freezer compartment must be used, with caution, for storing vaccines. The reason is that these units have cold spots and temperature fluctuations, and air circulating from the freezer could expose refrigerated vaccines in the same unit, to freezing temperatures.
Temperature Monitoring Devices (TMDs)
Every vaccine storage unit must have good TMD. CDC recommends the use of a continuous monitoring and recording device called a “digital data logger” (DDL), set at least every 30 minutes interval for recording. Many DDLs use a buffered temperature probe. Temperatures measured by a buffered probe match vaccine temperature more closely than those measured by standard thermometers, which tend to reflect air temperature. DDLs show details on how long a unit has been operating outside the recommended temperature range. Each DDL should have a current and valid Certificate of Calibration Testing (also known as a “Report of Calibration”) to certify it is accurate.
CDC recommends DDLs with the following characteristics:
- A detachable probe that best reflects vaccine temperatures
- Alarm for out-of-range temperatures
- Low-battery indicator
- Current, minimum, and maximum temperature display
- Recommended uncertainty of +/-0.5°C (+/-1°F)
- Logging interval (or reading rate) that can be set by the user to measure and record temperatures every 30 minutes
Temperature data from a DDL can be downloaded to a computer using an application or retrieved from a website for user review, which is crucial to vaccine safety. The app or website may also allow the user to preset the frequency of temperature readings.
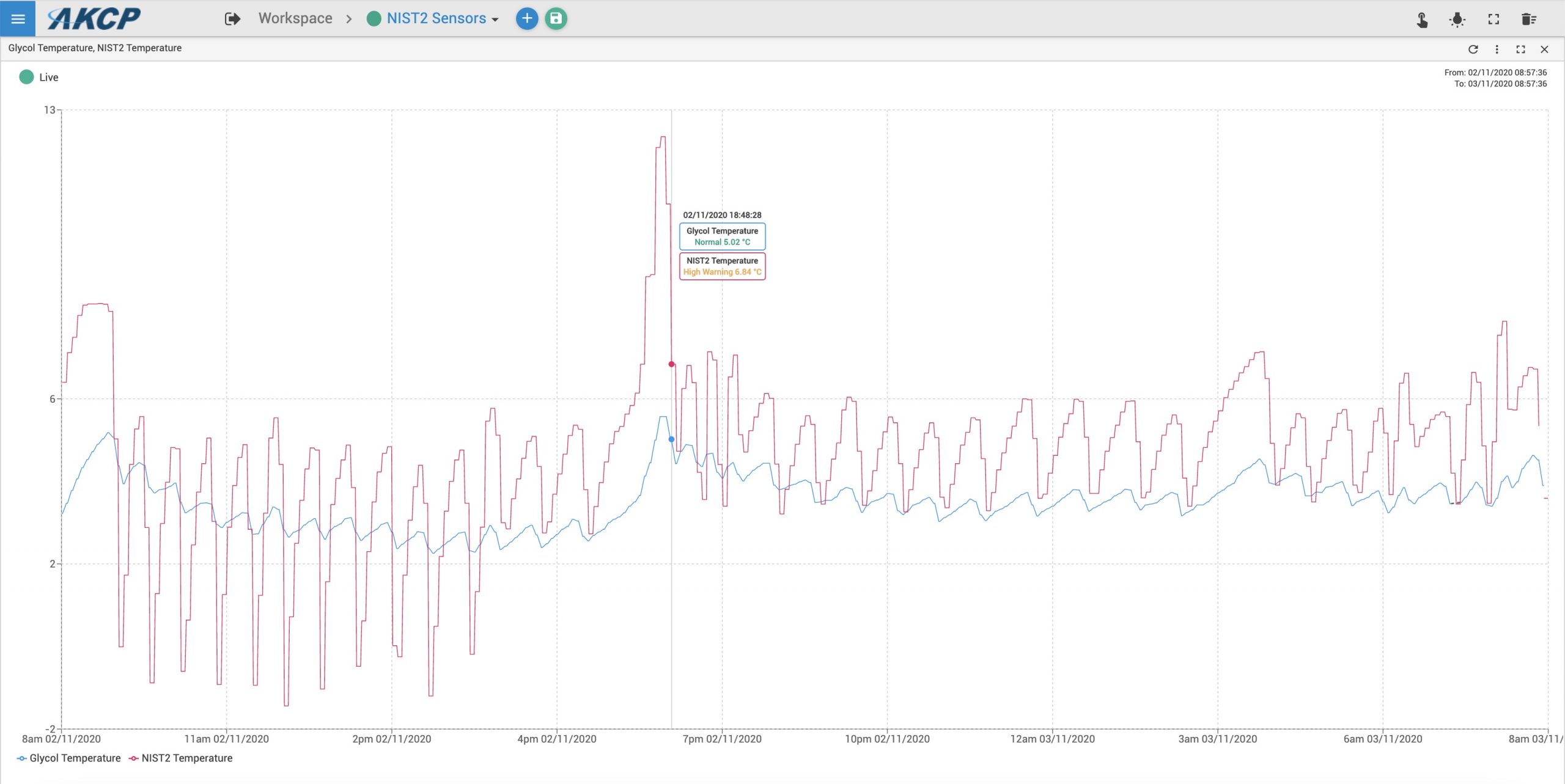
AKCP provide such temperature monitoring devices suitable for use in pharmaceutical industry and monitoring of medical refrigerators.

The AKCP Wireless Tunnel™ NIST2 and NIST4 type sensors are used with a glycol jar attachment to fulfill the requirements and guidelines from CDC, FDA and The WHO. Temperature data is graphed, reports can be generated, temperature alerts sent and vaccine batches tracked for temperature excursions.
Each facility should have a recommended TMD for:
Each vaccine storage unit
Each emergency transport unit
A backup TMD in case the primary device malfunctions or is out for calibration testing
Health facility cold chain equipment
Different levels within the national cold chain system require different types of equipment for transporting and storing vaccines and diluents within the prescribed temperature range.
- Primary level (national): the primary level generally uses cold or freezer rooms, freezers, refrigerators, cold boxes, and refrigerated trucks for transportation.
- Intermediate level (province or district): intermediate level generally uses cold and freezer rooms and/or freezers, refrigerators and cold boxes, and refrigerated trucks for transportation.
- Peripheral level (health center/facility or health post): health facilities generally need refrigerators (in certain instances with water pack freezing/cooling compartments), cold boxes, and vaccine carriers. In some countries, cold boxes alone may be used for monthly or weekly immunization sessions.
To ensure optimal performance, cold chain equipment used for immunization programs at any level must comply with the latest technological specifications, as prescribed with WHO prequalification standards or standards set by national regulatory authorities.
Cold chain equipment needed at peripheral-level health facilities.
Refrigerators
Health facility refrigerators are powered by electricity, solar energy, or gas (or kerosene). A health facility refrigerator should be chosen based according to the mode of power usage in the area and the combined capacity needed for vaccine and water pack storage.
Health facility refrigerator must never be tightly packed– always provide some space around the vaccines and diluents to allow air circulation, and easy access to the vaccine.
A health facility refrigerator must be able to hold:
- at least one month’s supply of vaccines and diluents in the refrigerator
- a one- or two-week reserve stock of vaccines and diluents (usually an additional 25–50% of the one-month supply)
- a minimum of four water packs in the freezer/cooling compartment
Cold boxes
A cold box is an insulated container that is lined with water packs to keep vaccines and diluents within the prescribed temperature range during transport. Cold boxes can store vaccines for periods of up to two days or more if there is no ready source of power. Once stored, cold boxes should not be opened until the vaccine is needed.

Monitoring cold chain temperatures
The data gathered from temperature monitoring devices must be recorded and analyzed on a regular basis to demonstrate that vaccines are being stored and transported at the correct temperatures.
In refrigerators, a standard manual temperature-recording chart is attached to the door of every vaccine refrigerator. Readings are taken twice a day, at least five days per week, preferably every day, including weekends and holidays. Daily readings should be taken from the same temperature monitoring device each time. Recording temperatures provides evidence that the refrigerator is being checked regularly. This identifies performance trends, sometimes even before automatic alarms pick them up.
AKCP provides a solution to monitor the complete cold chain, from manufacturer, through transportation, warehousing and last mile delivery.
![]()
Taking action when a vaccine refrigerator’s temperature is not within the prescribed limit;
If the temperature of the refrigerator is below +2 °C (32 °F), a report should be made to the supervisor.
Corrective action includes the following protocols:
- Turn the thermostat knob so the arrow points to a setting that will make the refrigerator warmer.
- Check whether the door of the freezer closes properly. The seal may be damaged. If broken, repair it.
- If the temperature has fallen below 0 °C for any length of time, check freeze-sensitive vaccines to see if they have been damaged by freezing, using the Shake Test.
Corrective action includes the following procedure:
- Make sure that the refrigerator is working. If it is not working, check whether the power supply is working.
- Check whether the door of the refrigerator or the freezing compartment closes properly; if the seal is broken, the temperature will fluctuate. Correct it.
- Check whether frost is preventing cold air in the freezing compartment from entering the refrigerator compartment. Defrost if necessary.
- If the power supply, door seal, and frost levels are all in working order, turn the thermostat knob so that the arrow points to a setting that will make the refrigerator cooler.
- If the temperature cannot be maintained between +2 °C and +8 °C (32 °F and 46.3°F), replace the equipment with another that can maintain the temperature range until the refrigerator is repaired.
Remember: to avoid freezing vaccines, do not adjust the thermostat to a cooler (higher number) setting after a power cut or when vaccines arrive.
Conclusions
Vaccine cold storage rooms have the latest technologies in cooling temperature to ensure the safety of the life science products. These temperatures are prescribed by the WHO or the concerned government agencies to safeguard the health of users.
Vaccines must be arranged inside cold chain equipment in a way that helps ensure that they remain in good condition, with minimum risk of exposure to damaging temperatures.
More details on AKCP solution specifically designed for the pharmaceutical industry can be found on https://www.parma-mon.com


